Details of the Drug
General Information of Drug (ID: DMVHKZT)
| Drug Name |
1,4-Butanediol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1,4-BUTANEDIOL; Butane-1,4-diol; 110-63-4; 1,4-Butylene glycol; Tetramethylene glycol; 1,4-Dihydroxybutane; 1,4-Tetramethylene glycol; Tetramethylene 1,4-diol; Sucol B; 1,4-BD; DIOL 14B; Agrisynth B1D; UNII-7XOO2LE6G3; HO(CH2)4OH; NSC 406696; CCRIS 5984; 1,4-Dihdyroxybutane; HSDB 1112; EINECS 203-786-5; HOCH2CH2CH2CH2OH; BRN 1633445; 7XOO2LE6G3; AI3-07553; CHEBI:41189; WERYXYBDKMZEQL-UHFFFAOYSA-N; MFCD00002968; DSSTox_CID_4666; DSSTox_RID_77492; DSSTox_GSID_24666; BDO; CAS-110-63-4; BU1; 1,4-Butanediol, homopolymer; 4-hydroxybutanol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
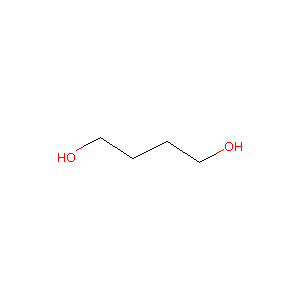 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 90.12 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
